

JUBLIA Topical Solution 10% w/w (HK-66407)
Topical treatment of onychomycosis (tinea unguium) of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes
-
Active Ingredient: Efinaconazole
-
Dosage: Once daily without debridement, 48 wks
-
Packing : 4ml/bottle
-
Manufacturer: Kaken Pharmaceutical Co., Ltd. (Japan)
-
Class: PoM
-
International Approvals: US, Canada, Japan, Korea, Taiwan
Videos
Mechanism of Action (3:16)
Therapeutic Efficacy
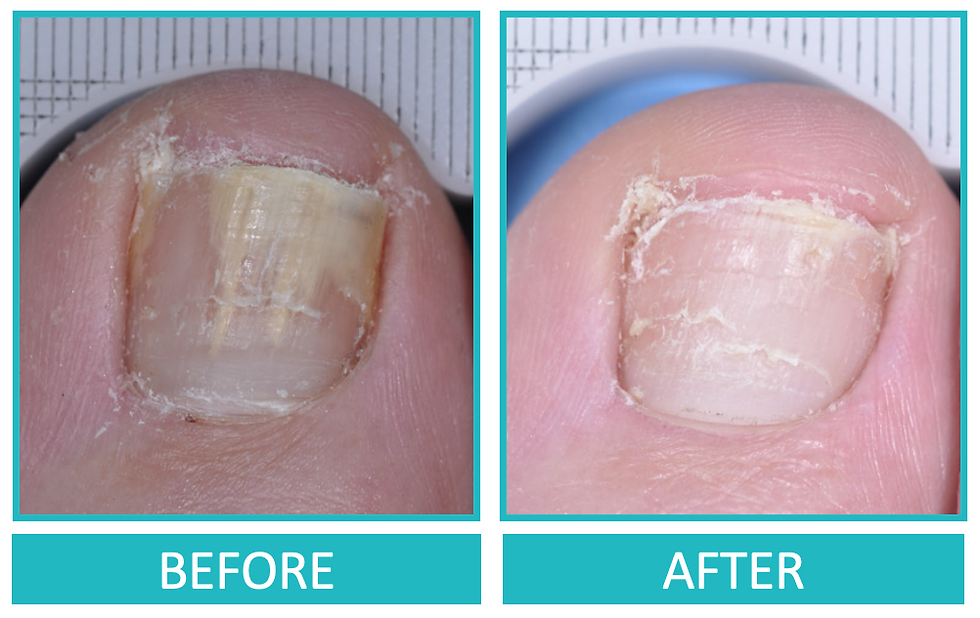



Individual results may vary
-
In multi-centre, randomised Phase III clinical trials including Asian population, JUBLIA monotherapy demonstrated significant efficacy for mycological cure, measured as negative microscopy and fungal culture.
-
Patients did not receive debridement.
The treatment aim for onychomycosis is to eliminate the fungus to produce a healthy nail.
Professional monitoring

Related literature
1. Gupta AK, et al. Efinaconazole: a new topical treatment for onychomycosis. Skin Therapy Lett. 2014 Jan-Feb;19(1):1-4.
2. Elewski BE, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: Two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013 Apr; 68(4):600-608.
3. Jo Siu WJ, et al. Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob Agents Chemother. 2013 Apr; 57(4):1610-6.
4. Vlahovic TC, et al. Efinaconazole Topical, 10% for the Treatment of Toenail Onychomycosis in Patients with Diabetes. J Drugs Dermatol. 2014; 13 (10): 1186-1190.
JUBLIA® Abbreviated Prescribing Information
Presentation: JUBLIA® (efinaconazole) topical solution, 10% w/w is a clear, colorless to pale yellow solution supplied in a white plastic bottle with an integrated flow-through brush applicator. Indications: Topical treatment of onychomycosis (tinea unguium) of the toenails due to Trichophyton rubrum and Trichophyton mentagrophytes. Dosage: Apply to affected toenails once daily for 48 weeks. When applying JUBLIA, ensure the toenail, the toenail folds, toenail bed, hyponychium, and the undersurface of the toenail plate, are completely covered. Contraindications: Hypersensitivity to efinaconazole or excipients or component of the container. Precautions: JUBLIA is for external use only and is not for ophthalmic, oral, or intravaginal use. Flammable, avoid use near heat or open flame. Drug Interactions: In vitro studies have shown that JUBLIA, at therapeutic concentrations, neither inhibits nor induces cytochrome P450 (CYP450) enzymes. Adverse Reactions: The most common adverse reactions (incidence >1%) were ingrown toenails, application site dermatitis, application site vesicles, and application site pain.
Before prescribing, please consult full prescribing information which is available upon request.
Learn More
Contact a medical representative
- Medical information
- Usage demo
- CME Seminar schedule
Tel. 2524 2462
Fax. 2860 1635



